Reauthorisation
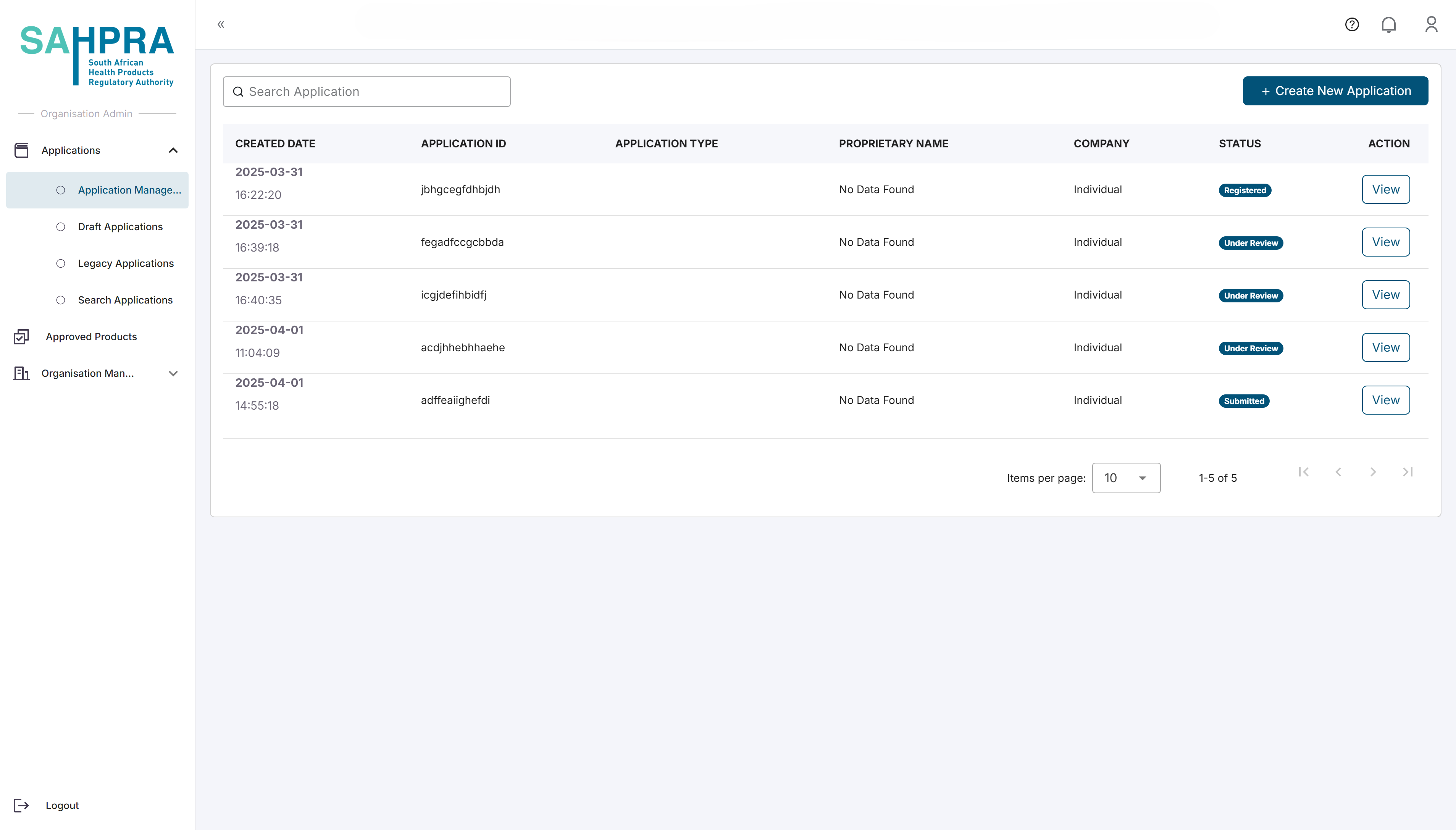
Overview
Reauthorisation are used to an Application for Section 21 that has already previously been submitted, but needs to be reauthorised to be used as a new application with the ame information again.
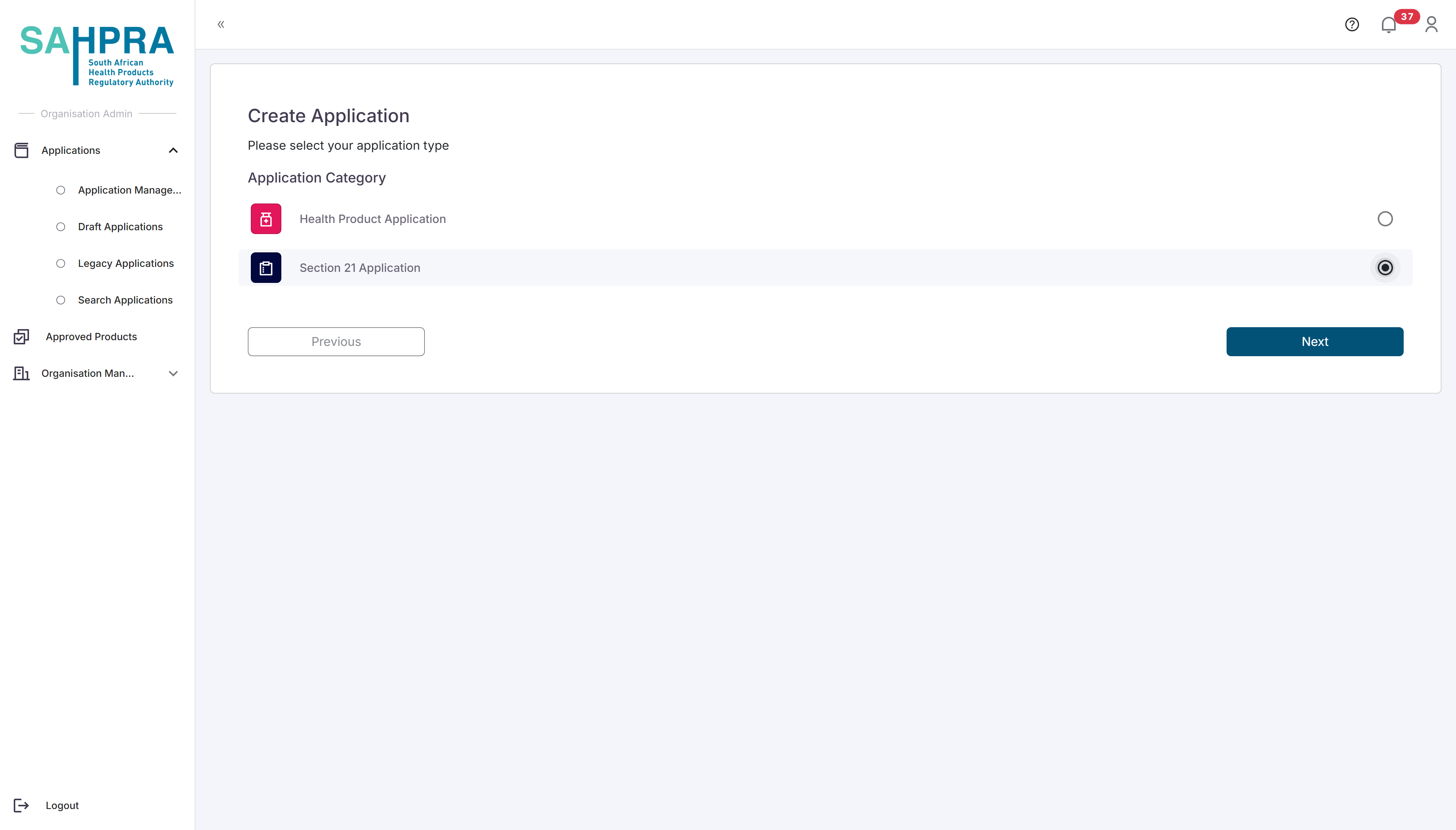
Step 2: Select Application Type
- Select “Section 21 Application” from the available application types.
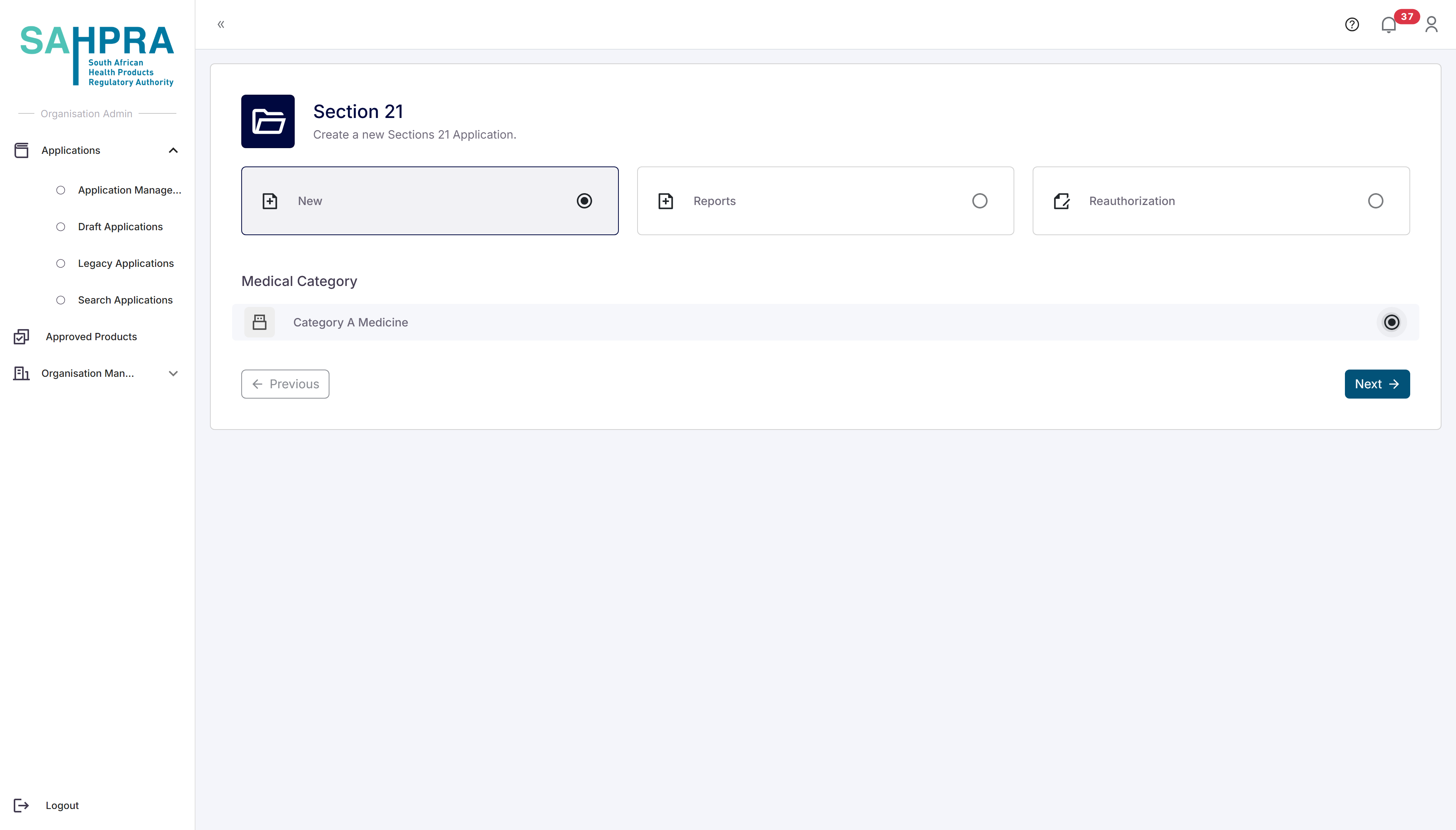
The options at the top section relate to the various options and in the case of Section 21 they are: New (new application), Reports (on an existing application) and Reauthorization (also on an existing application). In this case select “Reauthorization” where the following steps will ensue:
- Click “Next” to proceed.
Step 4: Review application
Once you are satisfied with the details and click the “Next” button the system will open the application with pre-filled data from the previous application. Note that the only details available for editing are only for changes on Prescription, Dosage and Patient consent.
Step 5: Upload Additional Documents
- Upload any additional documents that is relevant to your application
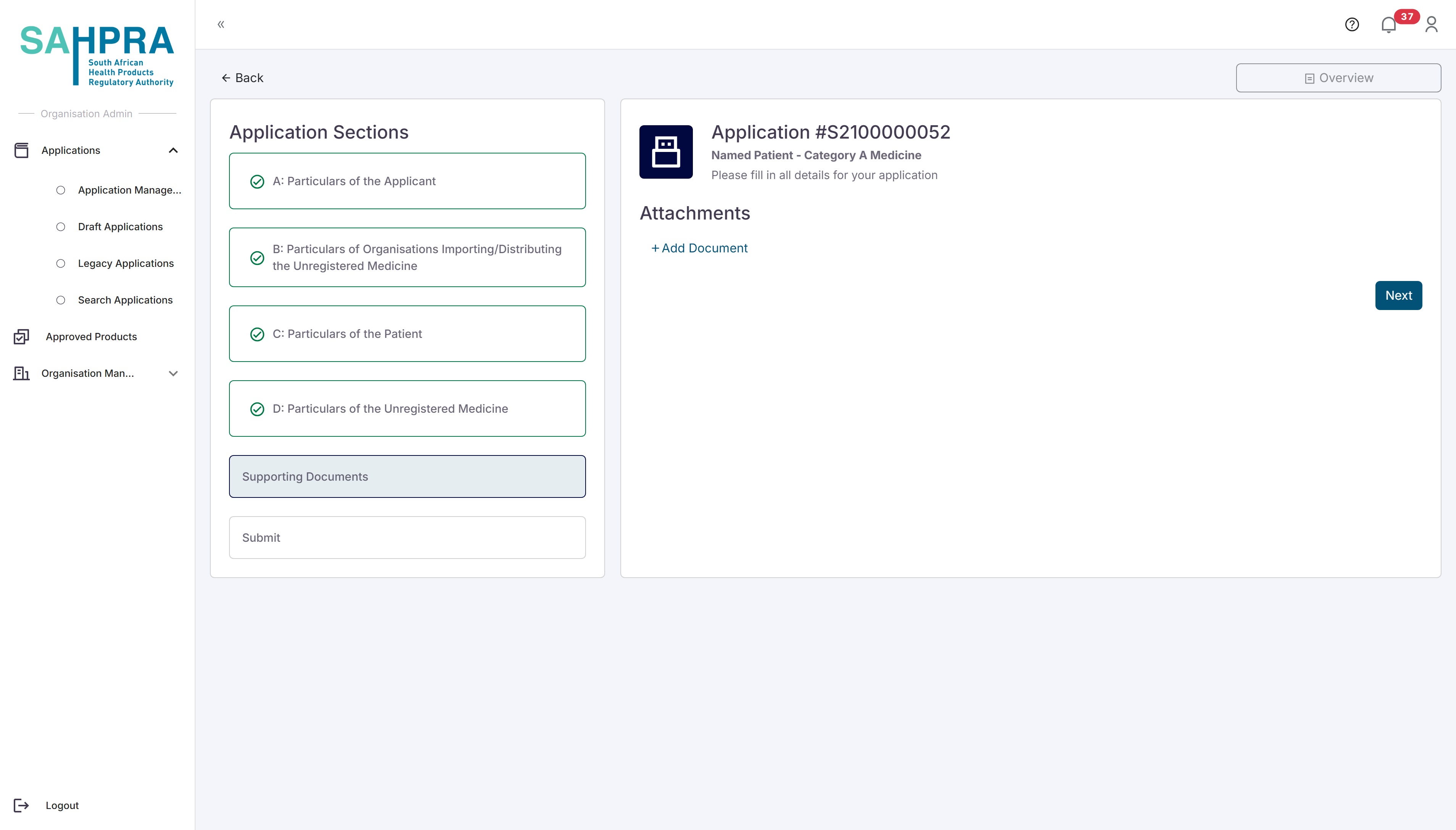
- Click “Next” to proceed.
Step 6: Review and Submit
Review all entered information for accuracy and completeness.
Click “Continue To Order” to proceed to payment.
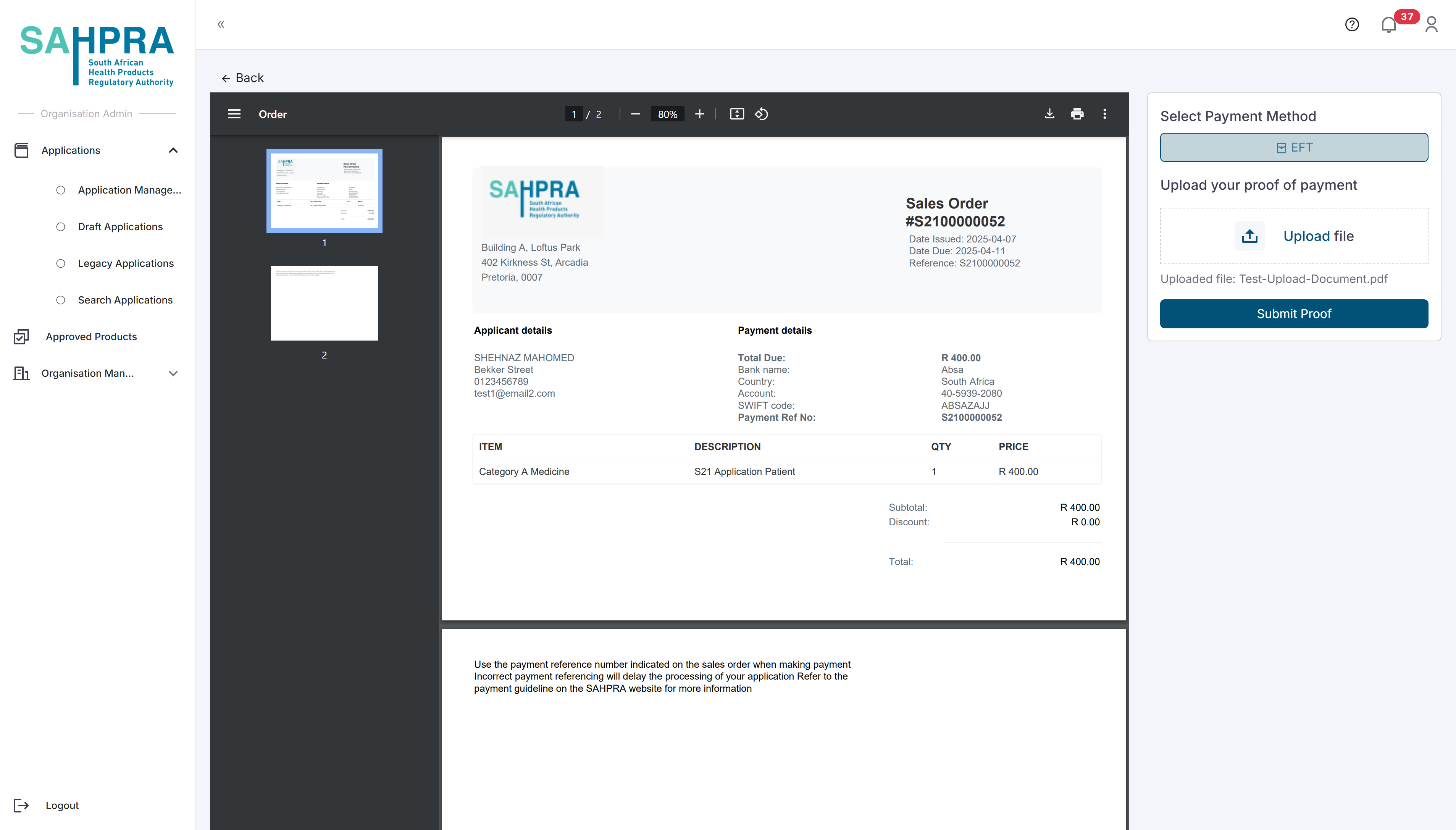
Step 7: Process Payment
Select “EFT” as the payment method.
Upload your proof of payment by clicking the upload button and selecting your file.
Click “Submit Proof” to submit your payment proof.
- You will be returned to the Applications Dashboard.