Application Export/Import tool (Retired)
Overview
The HPA Export/Import tool provides organizations with a streamlined process for managing application data. Through this tool, users can review, update, and correct application information, ensuring data accuracy and consistency across their records.
Key Features
- Export Applications Tab: A new Export Applications tab is available in the left-hand navigation menu. This tab allows users to:
- Review existing applications.
- Update missing or incomplete details, including indications, strength data, application numbers, and relationships between applications.
- Export application data for review and correction.
- Template Download: Users can download a blank template to create new applications not captured in legacy data. This is accessible via the Download Template button in the top-right corner of the Export Applications tab.
- The Application will remain in the same state with the same status after the data hve been imported
Important Guidelines
- Sequences with incorrect pricing due to missing data may be deleted directly within the exported spreadsheet.
- Do not add new sequences or submissions not part of the original application to the exported sheet. Instead, use the standard portal process for creating new submissions.
Application Data Sheet
- This sheet displays the overall application data, including submission and sequence structures. Each row represents an individual sequence.
- Sequences can be modified, added, or deleted as needed.
- To delete a sequence, select the entire row, right click, and select delete
- To add a sequence, copy an existing sequence row and paste it in the next available row, then modify the data to match the new sequence’s data you wish to add
- The ID column (Column A) is automatically generated for new entries and should not be manually edited.

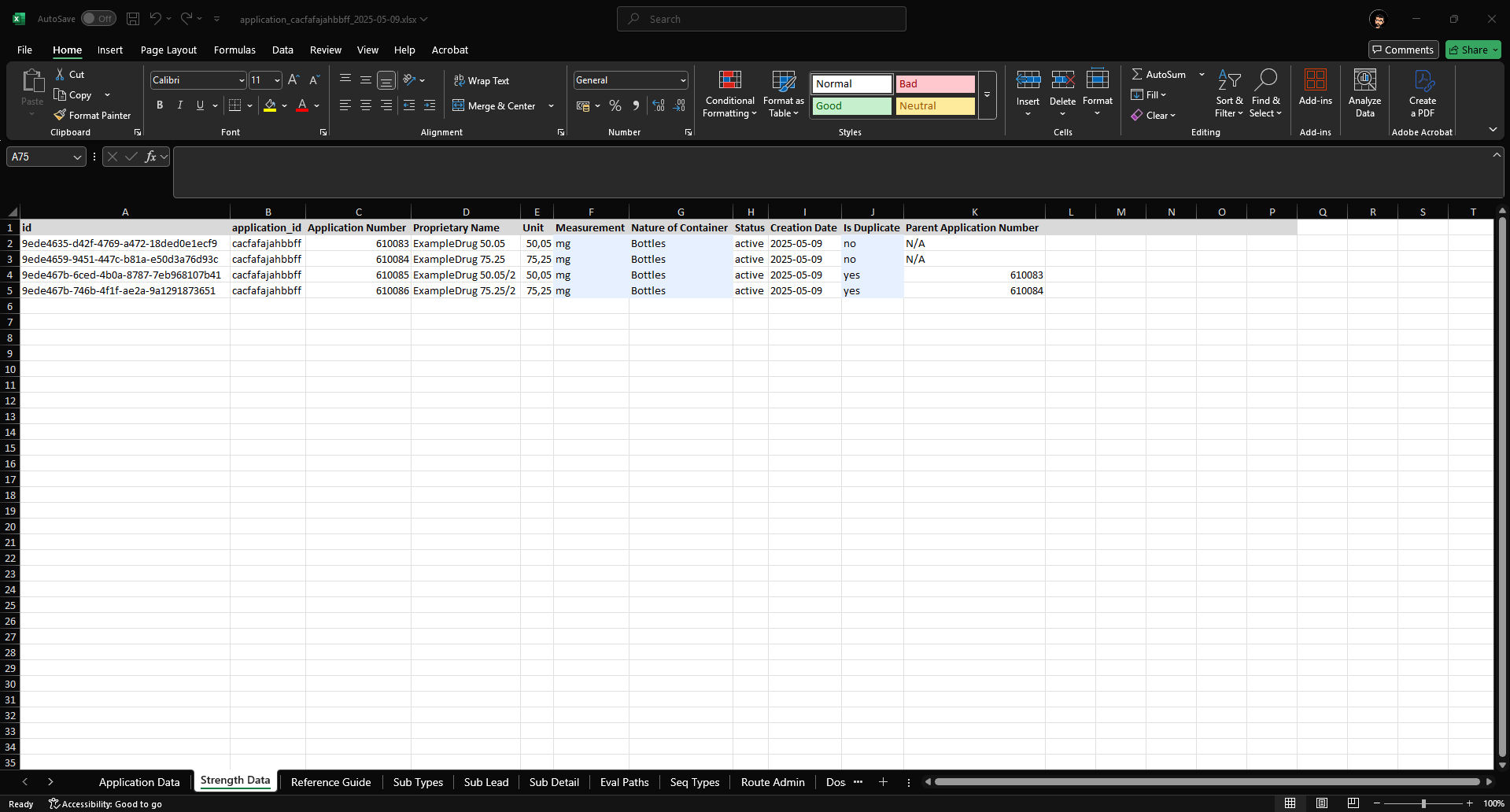
Strength Data Sheet
- This sheet allows users to modify strengths related to the application such as Proprietary Name, Unit Strength, Measurement, Nature of Container, and Duplicate Information.
- The “Application Number” (Column K) for a duplicate must reference the master Application number (Column C) it is associated with.
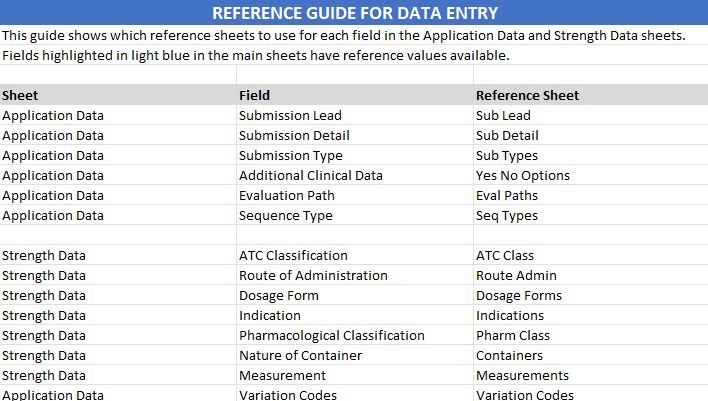
Reference Sheets
- Additional sheets within the exported file provide reference data to ensure accurate completion of application and strength data.
- Modifying these reference sheets will not affect data within the portal.
- Follow the instructions at the top of each sheet for guidance on proper data entry.
How to Use the HPA Export/Import Tool
- Navigate to the Export Applications tab in the portal.
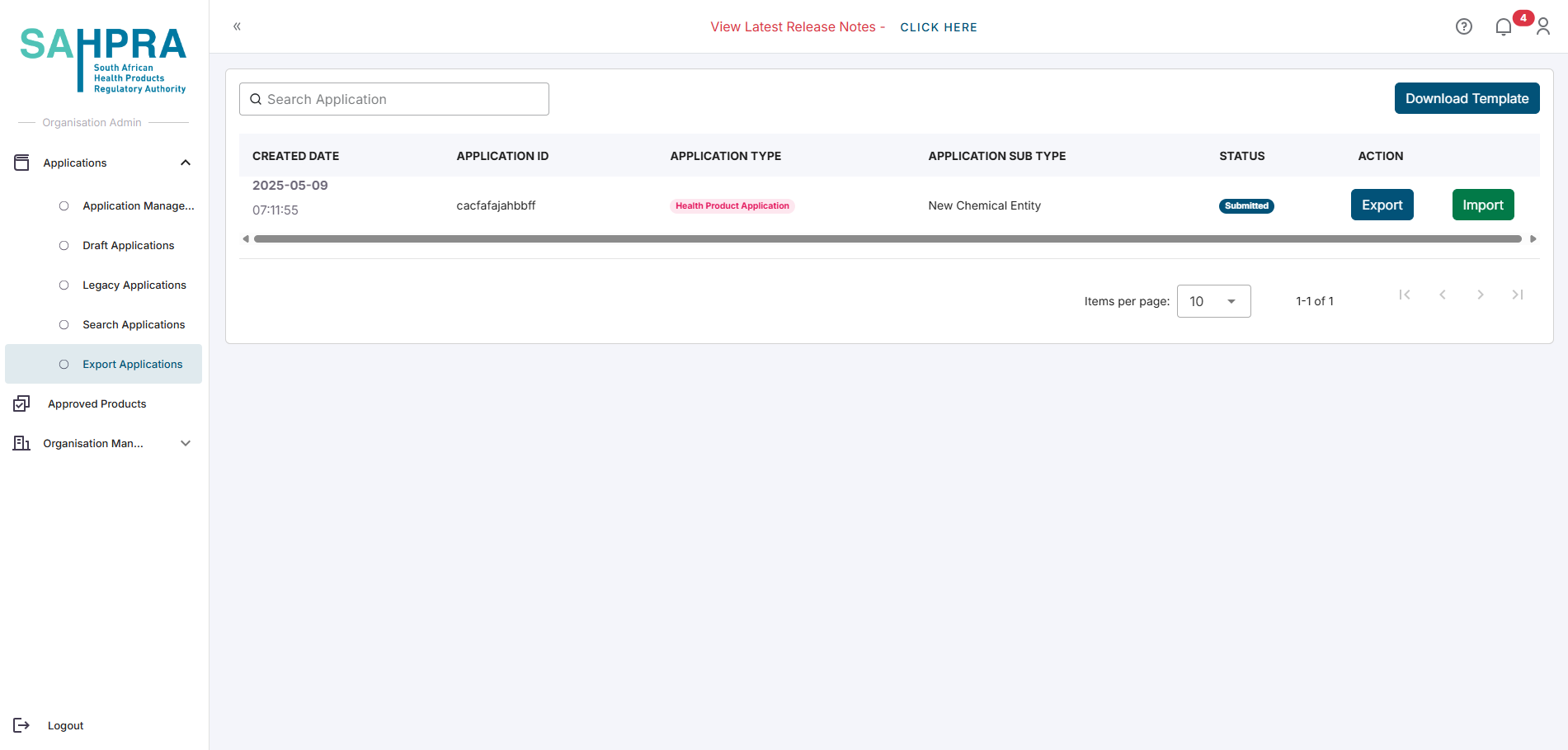
Locate the application you wish to amend.
Click Export next to the desired application to download the Excel file (This may take up to 30 seconds to export).
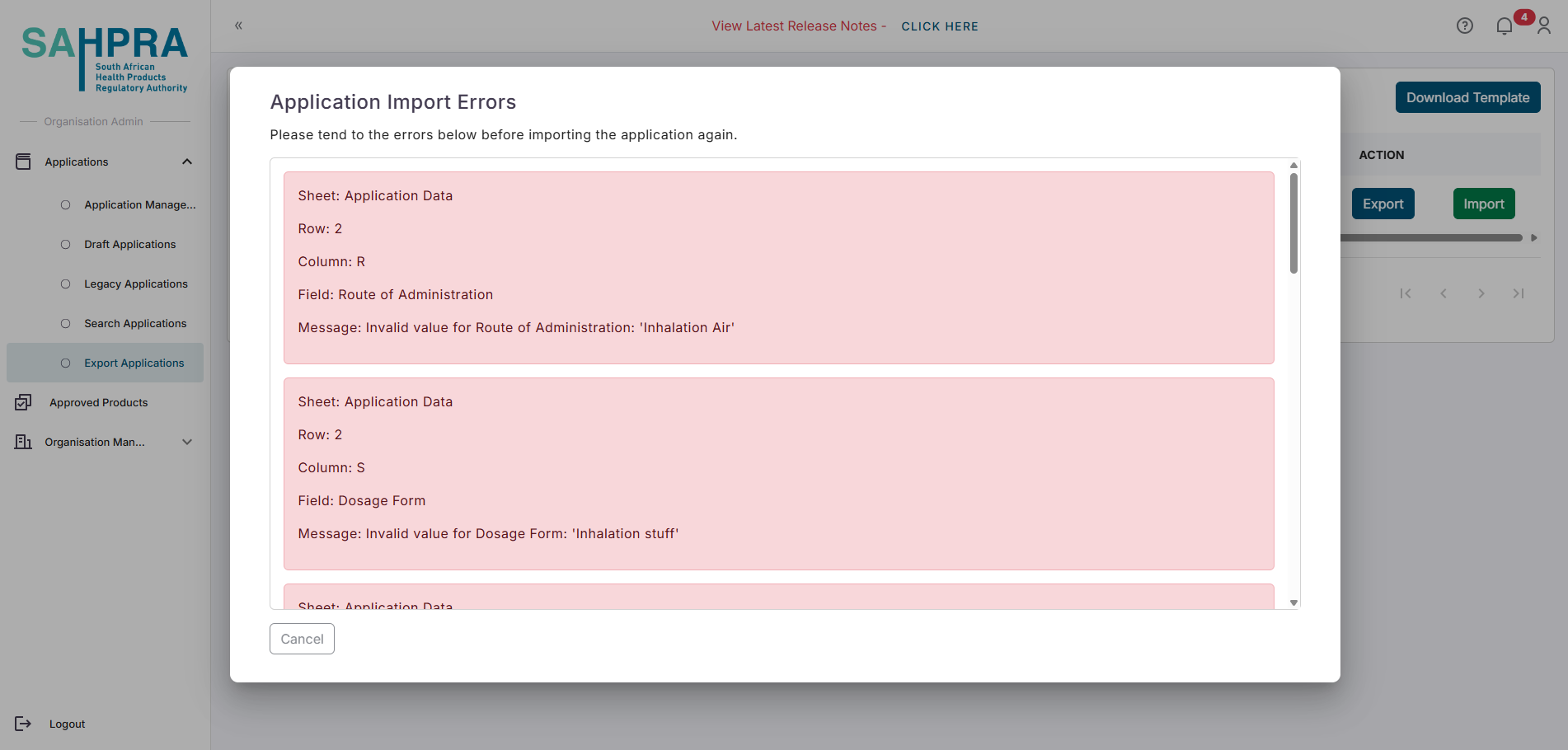
Review and update the application data within the exported file.
- If your data is not fully completed, you will get a popup message with a list of data still required to be completed
- Save your changes and import the file back into the portal, following any additional on-screen instructions.
Note: You can update and import your application data multiple times and can therefore change information again if needed
List of guidelines for each field entry
Sub Types
When filling out the Application Data sheet, you must select a valid Submission Type from this list. For example, if you’re submitting a new registration, choose ‘New Application’ or the appropriate type from this list. Do not use custom values that aren’t listed here.
Sub Lead
When filling out fields that reference this list, make sure to use only the exact values listed in the ‘Value’ column. This ensures data consistency and proper validation.
Sub Detail
When filling out fields that reference this list, make sure to use only the exact values listed in the ‘Value’ column. This ensures data consistency and proper validation.
Eval Paths
When filling out fields that reference this list, make sure to use only the exact values listed in the ‘Value’ column. This ensures data consistency and proper validation.
Seq Types
When filling out fields that reference this list, make sure to use only the exact values listed in the ‘Value’ column. This ensures data consistency and proper validation.
Route Admin
When filling out fields that reference this list, make sure to use only the exact values listed in the ‘Value’ column. This ensures data consistency and proper validation.
Dosage Forms
When filling out fields that reference this list, make sure to use only the exact values listed in the ‘Value’ column. This ensures data consistency and proper validation.
Containers
When filling out fields that reference this list, make sure to use only the exact values listed in the ‘Value’ column. This ensures data consistency and proper validation.
ATC Class
When filling out fields that reference this list, make sure to use only the exact values listed in the ‘Value’ column. This ensures data consistency and proper validation.
Indications
For the Indication field, you can select one or more values from this list, separated by pipe characters (|). For example: ‘Hypertension | Heart failure’. Multiple indications should be validated individually against this reference list.
Pharmacological Classification
When filling out fields that reference this list, make sure to use only the exact values listed in the ‘Value’ column. This ensures data consistency and proper validation.
Measurements
When specifying units in the Strength Data sheet, select an appropriate measurement unit from this list. For example, use ‘mg’ for milligrams, ‘ml’ for millilitres, etc.
Variation Codes
Variation codes follow a standardized format (e.g., B.III.1.a.2) and indicate the type of change being submitted.Each code corresponds to a specific type of change with a full description available in this reference sheet.
EXAMPLE FORMAT:
Code: (Q) B.II.e.3.a Description: Change in the shape or dimensions of the container or closure for non-sterile medicinal products
Code: B.I.b.1.c Description: Minor changes to the below approved test procedures
Multiple variation codes can be specified in the Variation Codes field, include both the code and description in the format shown above.
