Grouped Variations
This section guides you through the process of creating a grouped variation to an existing Health Product Application (HPA).
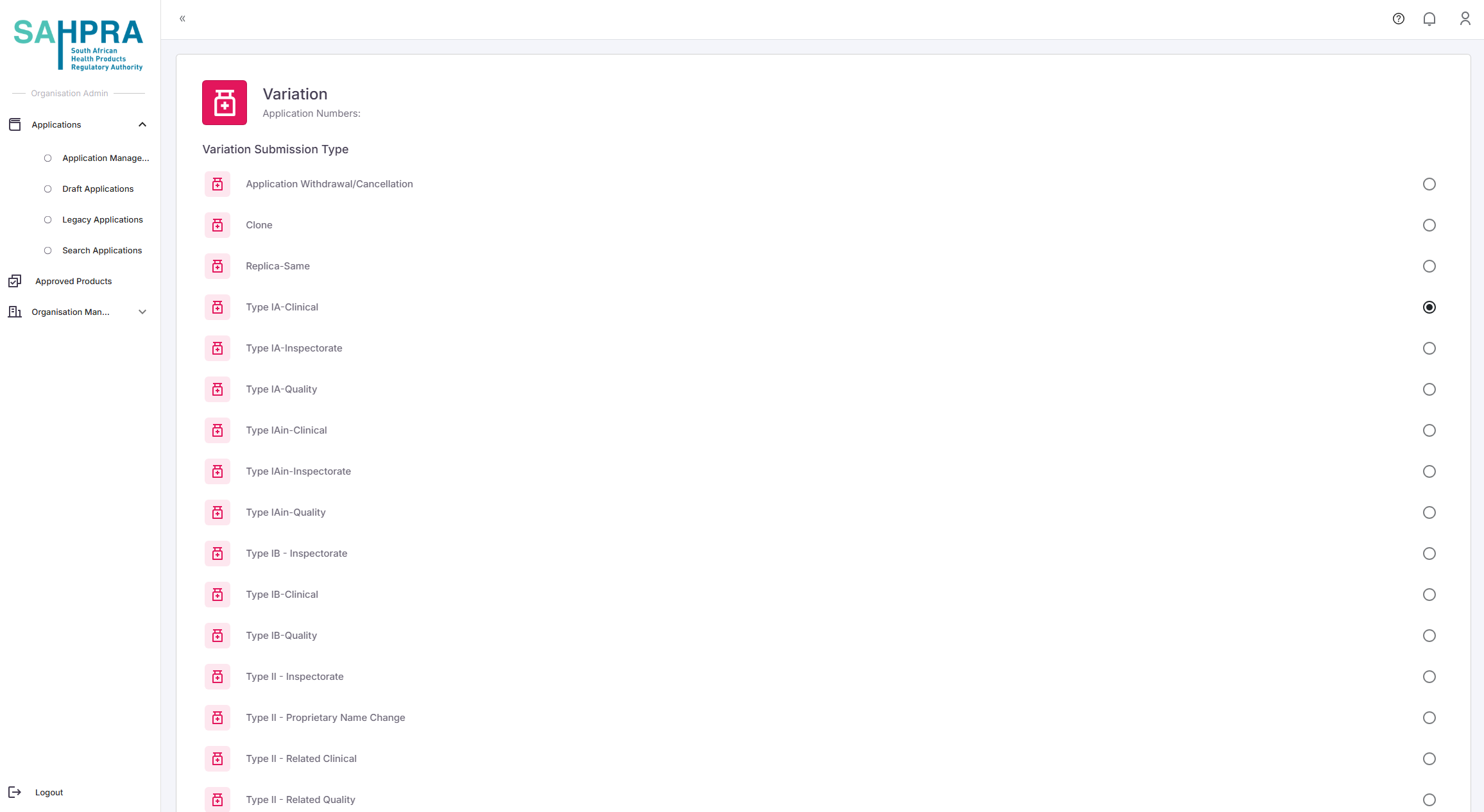
Grouped Variations are a combination of variations that can be done on an application consisting of IA,IB,IAIN and Type II variations. The applicant apply for Pre-Approval via the correct channels according to SAHPRA guidelines. Once the internal SAHPRA team grants an approval for variations you will receive a notification of the approval number to be entered on the applicable Grouped Variation submission type (either Clinical, Quality or Names & Scheduling). Once selecting the applicable variation submission type you will be able to select the approval number assigned to your organisation. Once selecting the approval number the pre-approved variation codes will be automatically populated on your variation application form. The standard process applies for completing the variation in terms of payment, sequence creation and FTP upload.
Prerequisites
Before beginning a variation application, ensure you have:
- The application ID of the existing registered product
- All required documentation for your specific variation types
- Details of the changes you wish to make
Step 1: Create New Variation
- Log in to the SAHPRA portal.
- You will be directed to the Applications Dashboard.
- Click the “Create New Application” button in the top right corner of the dashboard.
Step 2: Select Application Type
- Select “Health Product Application” from the available application types.
- Click “Next” to proceed.
Step 3: Select Variation Application Type
- Select the applicable Variation from the application types.
- Click “Next” to proceed.
Step 4: Link to Existing Application
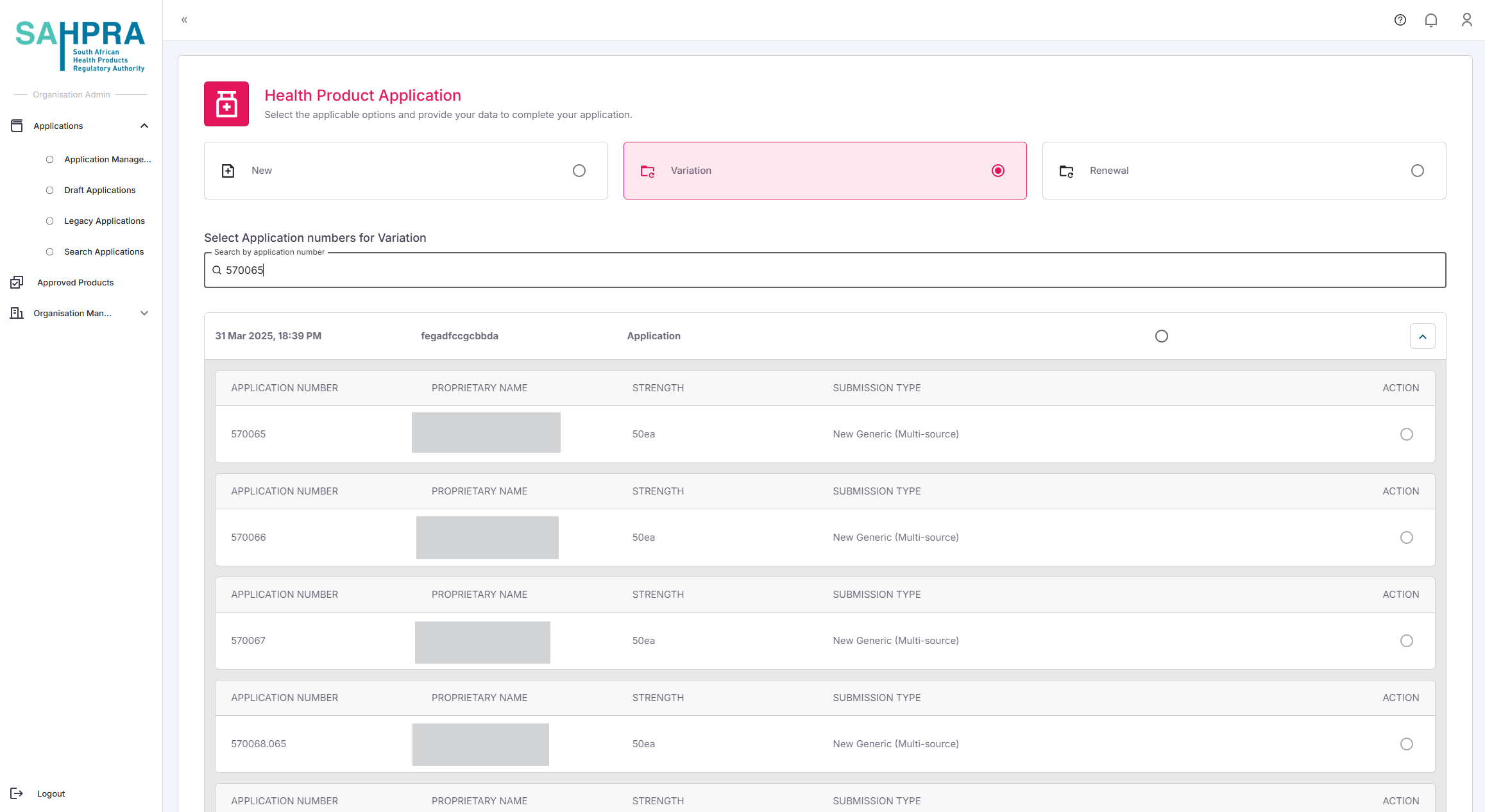
- Enter the Application ID of the existing product in the search field.
The system will display the matching application.
Select the application by clicking the checkbox next to it.
Click “Next” to proceed.
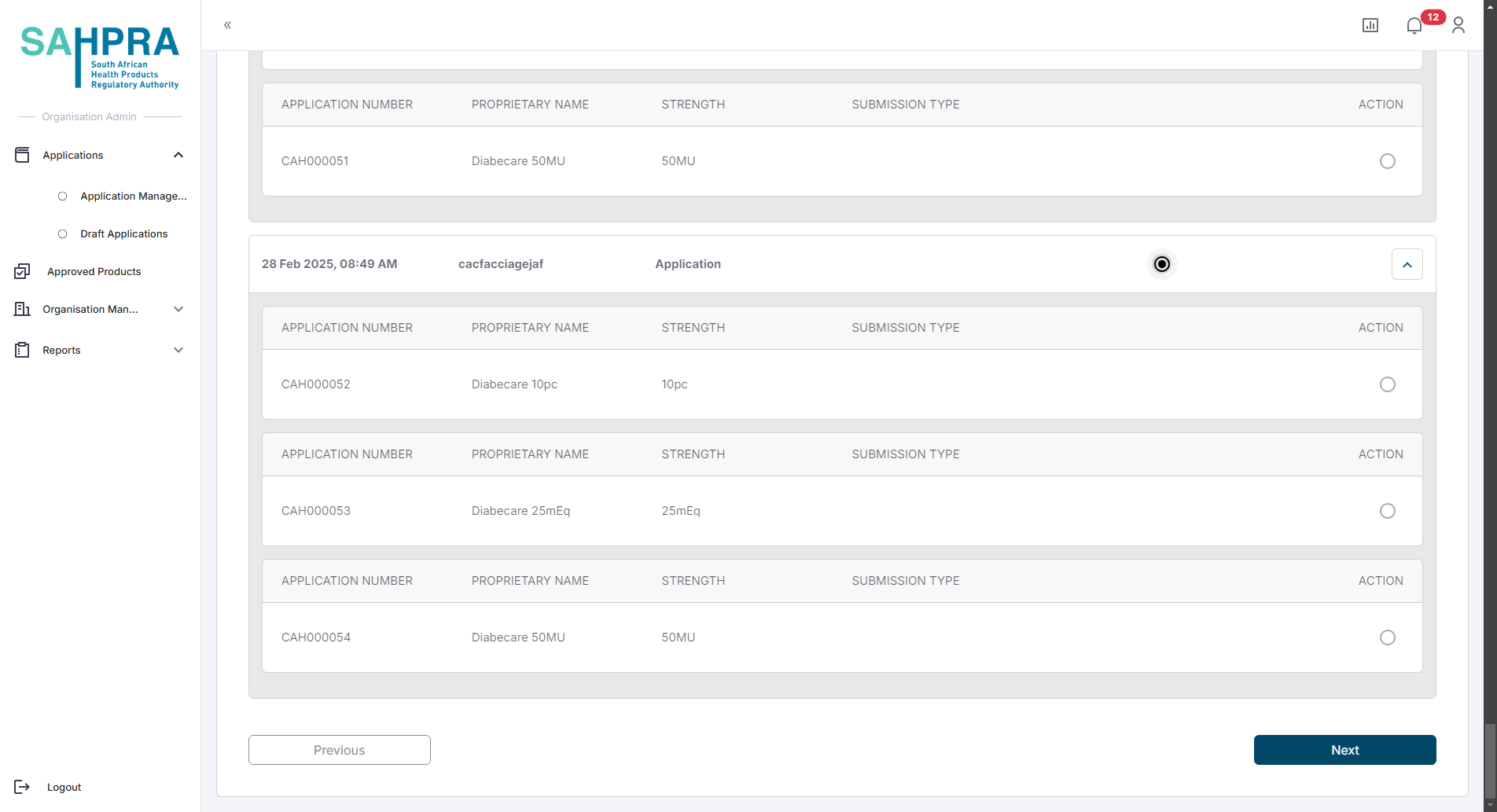
Step 5: Grouped Variations
Step 6: Create a New Sequence and complete payment
Once you are satisfied with the details and clicking the “Next” button a submission will be created, a sequence can be created, paid and submitted (Process for submission and sequence creation are described in detail in the “Creating a New Health Product Application” here). Once the application is approved the expiry date will be set to the applicable new expiry date.
Tips for Successful Variation Applications
- Ensure the variation code selected accurately reflects the changes you want to make.
- Provide comprehensive details in your variation description.
- Prepare all supporting documentation before starting the application.
- Double-check that you’ve linked to the correct existing application.
- Ensure your payment proof is clear and includes all required reference information.